
Only a photon with an energy of exactly 10.2 eV can. Welcome to the NIST Atomic Spectra Database, NIST Standard Reference Database 78. Cryogenic electron microscopy (cryo-EM) provides an effective way to investigate the structures of biological macromolecules (14). LeBeau and his research group used STEM to answer decades-old questions about relaxor ferroelectrics, a not-well-understood family of materials that. The atom absorbs or emits light in discrete packets called photons, and each photon has a definite energy. The photoelectric effect provided indisputable evidence for the existence of the photon and thus the particle-like behavior of electromagnetic radiation. After eliminating the problem for STEM, LeBeau focused on quantifying the atomic scale, or using STEM data to precisely measure the underlying crystal structure and chemistry of materials.
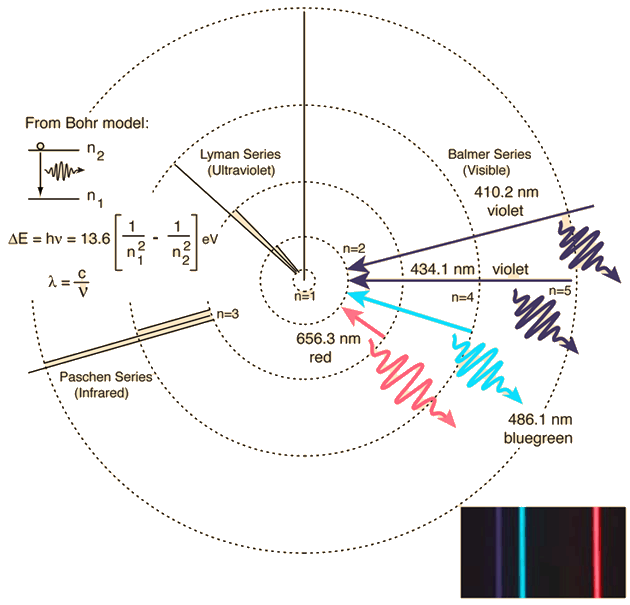
The energy is negative, indicating that the electron is bound to the nucleus where zero energy is equal to the infinite separation of the electron and proton. Because a hydrogen atom with its one electron in this orbit has the lowest possible energy, this is the ground state (the most stable arrangement of electrons for an element or a compound) for a hydrogen atom. To learn about the relationship between atomic spectra and the electronic structure of atoms. The energy scale of the atom, hcR, is equal to 13.6 electron volts. at a lower potential energy) when they are near each other than when they are far apart. With the Atomic Spectra: Light, Energy and Electron StructureChemTopic Lab Activity, learn to recognize continuous versus line emission spectra for light sources using a spectroscope, and use the spectroscope to observe the atomic spectra of different elements and identify elements present in a variety of light sources.

\) indicates that the electron-nucleus pair is more tightly bound (i.e.


 0 kommentar(er)
0 kommentar(er)
